IBU Tablets
Generic name:ibuprofen
Dosage form: tablet
Drug class:Nonsteroidal anti-inflammatory drugs
Medically reviewed by Drugs.com. Last updated on Mar 22, 2022.
On This Page
Cardiovascular Thrombotic Events
- Nonsteroidal anti-inflammatory drugs (NSAIDs) cause an increased risk of serious cardiovascular thrombotic events, including myocardial infarction and stroke, which can be fatal. This risk may occur early in treatment and may increase with duration of use. (See WARNINGS and PRECAUTIONS).
- IBU Tablets are contraindicated in the setting of coronary artery bypass graft (CABG) surgery (See CONTRAINDICATIONS and WARNINGS).
Gastrointestinal Risk
- NSAIDS cause an increased risk of serious gastrointestinaladverse events including bleeding, ulceration, and perforationof the stomach or intestines, which can be fatal. These eventscan occur at any time during use and without warning symptoms.Elderly patients are at greater risk for serious gastrointestinalevents. (See WARNINGS).
The IBU brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
IBU Tablets Description
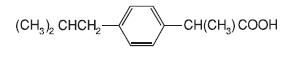
IBU Tablets contain the active ingredient ibuprofen, which is (±) -2 - (p - isobutylphenyl) propionic acid. Ibuprofen is a white powde rwith a melting point of 74-77° C and is very slightly soluble in water(<1 mg/mL) and readily soluble in organic solvents such as ethanol and acetone. The structural formula is represented below:
IBU, a n...



