Ibutilide Fumarate Injection
Dosage form: injection, solution
Drug class:Group III antiarrhythmics
Medically reviewed by Drugs.com. Last updated on Dec 1, 2020.
On This Page
Rx only
FOR INTRAVENOUS INFUSION ONLY
Ibutilide Fumarate Injection Description
Ibutilide Fumarate Injection is an antiarrhythmic drug with predominantly class III (cardiac action potential prolongation) properties according to the Vaughan Williams Classification. Each milliliter of Ibutilide Fumarate Injection contains 0.1 mg of ibutilide fumarate (equivalent to 0.087 mg ibutilide free base), 0.189 mg sodium acetate trihydrate, 8.9 mg sodium chloride, hydrochloric acid and/or sodium hydroxide to adjust pH to approximately 4.6, and water for injection.
Ibutilide Fumarate Injection is an isotonic, clear, colorless, sterile aqueous solution.
Ibutilide fumarate has one chiral center, and exists as a racemate of the (+) and (−) enantiomers.
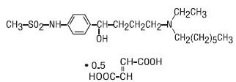
The chemical name for ibutilide fumarate is Methanesulfonamide, N-{4-{4-(ethylheptylamino)-1 -hydroxybutyl}phenyl}, (+)(−), (E)-2-butenedioate (1:0.5) (hemifumarate salt). Its molecular formula is C22H38N2O5S, and its molecular weight is 442.62.
Ibutilide fumarate is a white to off-white powder with an aqueous solubility of over 100 mg/mL at pH 7 or lower.
The structural formula is represented below:
Ibutilide Fumarate Injection - Clinical Pharmacology
Mechanism of Action
Ibutilide Fumarate Injection prolongs action potential duration in isolated adult cardiac myocytes and increases both atrial and ventricular refractoriness in vivo, i.e., class III electrophysiologic effects. Voltage clamp studies indicate that Ibutilide Fumarate Injection, at nanomolar concentrations, delays repolarization by acti...