Intal
Generic name:cromolyn sodium
Dosage form: Nebulizer Solution
Drug class:Mast cell stabilizers
Medically reviewed by Drugs.com. Last updated on Sep 21, 2021.
For Inhalation Use Only–Not for Injection
Rx only
On This Page
The Intal brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
Description
The active ingredient of Intal Nebulizer Solution is cromolyn sodium, USP. It is an inhaled anti-inflammatory agent for the preventive management of asthma. Cromolyn sodium is disodium 5,5'-[(2-hydroxytrimethylene)dioxy]bis[4-oxo-4H-1-benzopyran-2-carboxylate]. The empirical formula is C23H14Na2O11; the molecular weight is 512.34. Cromolyn sodium is a water soluble, odorless, white, hydrated crystalline powder. It is tasteless at first, but leaves a slightly bitter aftertaste. Intal Nebulizer Solution is clear, colorless, sterile, and has a target pH of 5.5.
The molecular structure is:
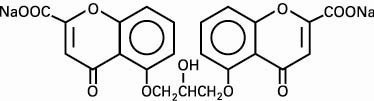
Each 2 mL ampule of Intal Nebulizer Solution (cromolyn sodium inhalation solution, USP) contains 20 mg cromolyn sodium, USP, in purified water.
Clinical Pharmacology
In vitro and in vivo animal studies have shown that cromolyn sodium inhibits sensitized mast cell degranulation which occurs after exposure to specific antigens. Cromolyn sodium acts by inhibiting the release of mediators from mast cells. Studies show that cromolyn sodium indirectly blocks calcium ions from entering the mast cell, thereby preventing mediator release.
Cromolyn sodium inhibits both the immediate and non-immediate bronchoconstrictive reactions to inhaled antigen. Cromolyn sodium also attenuates bronchospasm caused by exercise, toluene diisocyanate, aspirin, cold air, sulfur dioxide, and environmental pollutants.
Cromolyn sodium has no intrinsic bronchodilator or antihistamine activity.
After administration by inhalation, approximately 8% of the total cromolyn...



