Ionosol and Dextrose
Generic name: dextrose monohydrate, sodium lactate, potassium chloride, magnesium chloride, potassium phosphate, monobasic and sodium phosphate, monobasic, monohydrate
Dosage form: injection
Drug class:Intravenous nutritional products
Medically reviewed by Drugs.com. Last updated on Oct 22, 2021.
On This Page
(MULTIPLE ELECTROLYTES AND 5% DEXTROSE INJECTION TYPE 1, USP)
A MAINTENANCE ELECTROLYTE SOLUTION
Flexible Plastic Container
Rx only
Ionosol and Dextrose Description
Ionosol MB and 5% Dextrose Injection (Multiple Electrolytes and 5% Dextrose Injection Type 1, USP) is a sterile, nonpyrogenic solution designed for intravenous administration. The solution is formulated to provide fluid and electrolytes for treatment of dehydration and acidosis.
Each 100 mL contains dextrose, hydrous 5 g; sodium lactate, anhydrous 260 mg; potassium chloride 141 mg; magnesium chloride, hexahydrate 30 mg; monobasic potassium phosphate, anhydrous 15 mg; and monobasic sodium phosphate, monohydrate 25 mg.
Each liter contains 25 mEq sodium (Na+); 20 mEq potassium (K+); 3 mEq magnesium (Mg++); 22 mEq chloride (Cl¯); 3 mM of phosphate (PO4≡); and 23 mEq lactate (CH3CH(OH)COO¯).
The electrolyte content is hypotonic (100 mOsmol/L) in relation to the extracellular fluid (approx. 280 mOsmol/L). The osmolarity for the total solution is 352 mOsmol/L (calc.). May contain hydrochloric acid for pH adjustment. pH is 5.0 (4.0 to 6.5).
Dextrose, USP, hydrous is chemically designated C6H12O6 • H2O (D-glucose, monohydrate), a hexose sugar freely soluble in water. Dextrose, hydrous has the following structural formula:
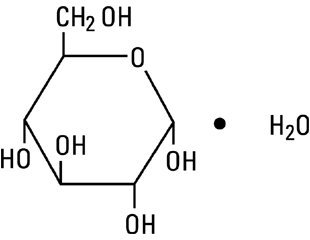
Magnesium Chloride, USP, hexahydrate is chemically designated MgCl2 • 6H2O, colorless flakes or crystals very soluble in water.
Potassium Chloride, USP is chemically designated KCl, a white granular powder freely soluble in water.
Monobasic Potassium Phosphate, NF, anhydrous is chemically designated ...



