Isopto Cetamide
Generic name:sulfacetamide sodium
Dosage form: ophthalmic solution
Drug class:Ophthalmic anti-infectives
Medically reviewed by Drugs.com. Last updated on Oct 22, 2021.
On This Page
The Isopto Cetamide brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
DESCRIPTION
Isopto Cetamide® (sulfacetamide sodium ophthalmic solution USP) 15% and CETAMIDE™ (sulfacetamide sodium ophthalmic ointment, USP) 10% are sterile topical antibacterial agents for ophthalmic use. The active ingredient is represented by the following structural formula:
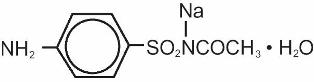
Chemical name: N-Sulfanilylacetamide monosodium salt monohydrate.
Each mL of solution contains: Active: Sulfacetamide sodium 15% (150 mg/mL). Preservative: Methylparaben 0.05%, Propylparaben 0.01%. Vehicle: 0.5% Hydroxyporpyl Methylcellulose 2910 (viscosity type, 4000 cps). Inactive: Sodium Thiosulfate 0.3%, Dibasic Sodium Phosphate and/or Monobasic Sodium Phosphate (to adjust pH), Purified Water.
pH range between 7.0 and 7.8 DM-02
Each gram of ointment contains: Active: Sulfacetamide Sodium 10% (100 mg). Preservatives: Methylparaben 0.05%, Propylparaben 0.01%. Inactive: White Petrolatum, Anhydrous Liquid Lanolin, Mineral Oil. DM-00
CLINICAL PHARMACOLOGY
Microbiology
The sulfonamides are bacteriostatic agents and the spectrum of activity is similar for all. Sulfonamides inhibit bacterial synthesis of dihydrofolic acid by preventing the condensation of the pteridine with aminobenzoic acid through competitive inhibition of the enzyme dihydropteroate synthetase. Resistant strains have altered dihydropteroate synthetase with reduced affinity for sulfonamides or produce increased quantities of aminobenzoic acid.
Topically applied sulfonamides are considered active against susceptible strains of the following common bacterial eye pathogens:



