Jencycla
Generic name:norethindrone
Dosage form: tablet
Drug classes:Contraceptives, Progestins
Medically reviewed by Drugs.com. Last updated on Apr 1, 2022.
On This Page
Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
Jencycla Description
Each tablet contains 0.35 mg norethindrone. Inactive ingredients include D&C yellow No. 10, FD&C blue No. 1, lactose anhydrous, magnesium stearate, povidone and sodium starch glycolate.
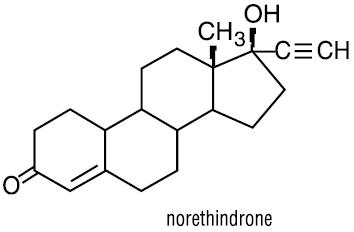
The chemical name of norethindrone is 17-hydroxy-19-nor-17α-pregn-4-en-20-yn-3-one. It has a molecular formula of C20H26O2 and a molecular weight of 298.4. It has the following structural formula:
Jencycla meets USP Dissolution Test 2.
Jencycla - Clinical Pharmacology
Jencycla progestin-only oral contraceptives prevent conception by suppressing ovulation in approximately half of users, thickening the cervical mucus to inhibit sperm penetration, lowering the midcycle LH and FSH peaks, slowing the movement of the ovum through the fallopian tubes, and altering the endometrium.
2. Pharmacokinetics
Serum progestin levels peak about two hours after oral administration, followed by rapid distribution and elimination. By 24 hours after drug ingestion, serum levels are near baseline, making efficacy dependent upon rigid adherence to the dosing schedule. There are large variations in serum levels among individual users. Progestin-only administration results in lower steady-state serum progestin levels and a shorter elimination half-life than concomitant administration with estrogens.
Indications and Usage for Jencycla
Progestin-only oral contraceptives are ...



