Larin Fe 1.5/30
Generic name:norethindrone acetate/ethinyl estradiol and ferrous fumarate
Dosage form: tablets
Drug classes:Contraceptives, Sex hormone combinations
Medically reviewed by Drugs.com. Last updated on Oct 22, 2021.
On This Page
(Each yellow tablet contains 1.5 mg norethindrone acetate and 30 mcg ethinyl estradiol. Each brown tablet contains 75 mg ferrous fumarate.)
Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
Larin Fe 1.5/30 Description
Larin Fe 1.5/30 is progestogen-estrogen combination.
Larin Fe 1.5/30: Each provides a continuous dosage regimen consisting of 21 oral contraceptive tablets and seven ferrous fumarate tablets. The ferrous fumarate tablets are present to facilitate ease of drug administration via a 28-day regimen, are non-hormonal, and do not serve any therapeutic purpose.
Each yellow tablet contains norethindrone acetate (17 alpha-ethinyl-19-nortestosterone acetate), 1.5 mg; ethinyl estradiol (17 alpha-ethinyl-1,3,5(10)-estratriene-3, 17 beta-diol), 30 mcg. Also contains polyvinyl alcohol, titanium dioxide, talc, macrogol/polyethylglycol 3350 NF,lecithin (soya), iron oxide yellow, FD&C Blue No.2 Aluminum Lake, D&C Yellow No.10 Aluminum Lake,FD&C Yellow No.6 Aluminum Lake, lactose, magnesium stearate and pregelatinized corn starch.
The structural formulas are as follows:
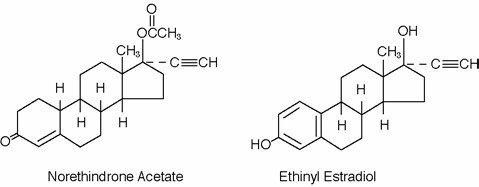
Each brown placebo tablet contains ferrous fumarate, polyvinyl alcohol,Talc, macrogol/polyethyleneglycol 3350 NF, lecithin (soya),iron oxide black ,iron oxide yellow, microcrystalline cellulose,hydroxypropyl cellulose, magnesium stearate and crospovidone.
Larin Fe 1.5/30 - Clinical Pharmacology
Combination oral contraceptives act by suppression of gonadotropins. Although the primary mechanism of this action is inhibition of ovulation, other alterations include changes in the cervical mucus (which increase the difficulty of sperm entry into the uterus) and the endometrium (which reduce the likelihood of implantation).



