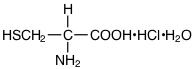L-Cysteine Hydrochloride Injection
Dosage form: injection, solution
Drug class:Intravenous nutritional products
Medically reviewed by Drugs.com. Last updated on Jul 22, 2021.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
On This Page
L-Cysteine Hydrochloride Injection Description
L-Cysteine Hydrochloride Injection, USP, 50 mg/mL, is a sterile, nonpyrogenic solution. Each mL contains: 50 mg of L-Cysteine Hydrochloride Monohydrate USP; Water for Injection, USP q.s.; Air replaced with Nitrogen. pH 1.0-2.5
L-Cysteine is a sulfur-containing amino acid. In premixed solutions of crystalline amino acids, cysteine is relatively unstable over time, eventually converting to insoluble cystine. To avoid such precipitation, L-Cysteine Hydrochloride Injection USP is intended to be used as an additive with Crystalline Amino Acid Injections immediately prior to administration to the patient.
The structural formula of Cysteine Hydrochloride Monohydrate USP is:
Molecular Weight | Molecular Formula |
175.63 | C3H7NO2S•HCl•H2O |
L-Cysteine Hydrochloride Injection - Clinical Pharmacology
L-Cysteine is synthesized from methionine via the trans-sulfuration pathway in the adult, but newborn infants lack the enzyme necessary to effect this conversion. Therefore, L-Cysteine is generally considered to be an essent...