Levlite
Generic name:levonorgestrel and ethinyl estradiol
Dosage form: tablets
Drug class:Contraceptives
Medically reviewed by Drugs.com. Last updated on Aug 23, 2021.
On This Page
Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
Rx only
The Levlite brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
Levlite Description
Each cycle of Levlite® 28 (levonorgestrel and ethinyl estradiol tablets, USP) consists of 21 pink active tablets each containing 0.100 mg levonorgestrel and 0.020 mg ethinyl estradiol; and seven white tablets—inert. The inactive ingredients are Calcium Carbonate USP, Corn Starch NF, Ferric Oxide/red/E 172 NF, Ferric Oxide/yellow/E 172 NF, Glycerin 85% Ph. Eur./DAB, Lactose Monohydrate NF, Magnesium Stearate NF, Montanglycol Wax (Wax E) DAB, Polyethylene glycol 6,000 NF, Povidone 25,000 USP, Povidone 700,000 USP, Pregelatinized Starch NF (Modified Starch), Sucrose NF, Talc USP and Titanium Dioxide, E 171 USP.
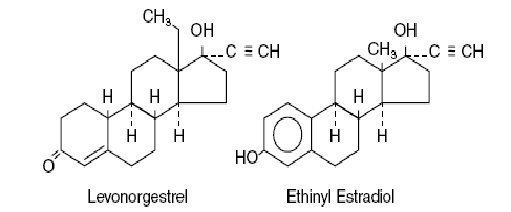
Levonorgestrel has a molecular weight of 312.4 and a molecular formula of C21H28O2. Ethinyl estradiol has a molecular weight of 296.4 and a molecular formula of C20H24O2. The structural formulas are as follows:
Levlite - Clinical Pharmacology
Combination oral contraceptives act by suppression of gonadotropins. Although the primary mechanism of this action is inhibition of ovulation, other alterations include changes in the cer...



