Aminohippurate Sodium
Dosage form: injection, solution
Drug class:Miscellaneous uncategorized agents
Medically reviewed by Drugs.com. Last updated on Dec 22, 2021.
On This Page
Aminohippurate Sodium Description
Aminohippurate Sodium1 is an agent to measure effective renal plasma flow (ERPF). It is the sodium salt of para-aminohippuric acid, commonly abbreviated “PAH”. It is water soluble, lipid-insoluble, and has a pKa of 3.83. The empirical formula of the anhydrous salt is C9H9N2NaO3 and its structural formula is:
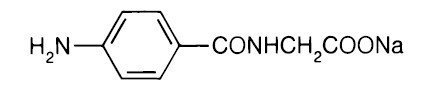
It is provided as a sterile, non-preserved 20 percent aqueous solution for injection, with a pH of 6.7 to 7.6. Each 10 mL contains: Aminohippurate Sodium 2 g. Inactive ingredients: Sodium hydroxide to adjust pH, water for injection, q.s.
- 1
Formerly referred to as Sodium para-Aminohippurate.
Aminohippurate Sodium - Clinical Pharmacology
PAH is filtered by the glomeruli and is actively secreted by the proximal tubules. At low plasma concentrations (1.0 to 2.0 mg/100 mL), an average of 90 percent of PAH is cleared by the kidneys from the renal blood stream in a single circulation. It is ideally suited for measurement of ERPF since it has a high clearance, is essentially nontoxic at the plasma concentrations reached with recommended doses, and its analytical determination is relatively simple and accurate.
PAH is also used to measure the functional capacity of the renal tubular secretory mechanism or transport maximum (TmPAH). This is accomplished by elevating the plasma concentration to levels (40-60 mg/100 mL) sufficient to saturate the maximal capacity of the tubular cells to secrete PAH.
Inulin clearance is generally measured during TmPAH determinations since glomerular filtration rate (GFR) must be known before calculations of secretory Tm measurements can be done (see DOSAGE AND ADMINISTRATION, Calculations).



