Lidotor Cream
Generic name:lidocaine and prilocaine
Dosage form: cream, kit
Drug class:Topical anesthetics
Medically reviewed by Drugs.com. Last updated on Dec 1, 2021.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. Read further information about unapproved drugs.
On This Page
Lidotor Cream Description
Lidocaine and Prilocaine Cream USP, 2.5%/2.5% is an emulsion in which the oil phase is a eutectic mixture of lidocaine and prilocaine in a ratio of 1:1 by weight. This eutectic mixture has a melting point below room temperature and therefore both local anesthetics exist as a liquid oil rather than as crystals. It is packaged in 5 gram and 30 gram tubes. Lidocaine is chemically designated as acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl), has an octanol: water partition ratio of 43 at pH 7.4, and has the following structure:
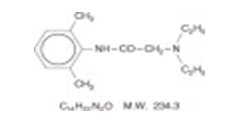
| C 14H 22N 2O | M.W. 234.3 | |
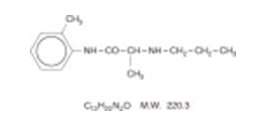
Prilocaine is chemically designated as propanamide, N-(2-methylphenyl)-2-(propylamino), has an octanol: water partition ratio of 25 at pH 7.4, and has the following structure: