Livostin
Generic name:levocabastine hydrochloride
Dosage form: Ophthalmic Suspension
Drug class:Ophthalmic antihistamines and decongestants
Medically reviewed by Drugs.com. Last updated on Dec 22, 2021.
Livostin®
0.05% (levocabastine hydrochloride ophthalmic suspension)
On This Page
Livostin Description
Livostin™ 0.05% (levocabastine hydrochloride ophthalmic suspension) is a selective histamine H1-receptor antagonist for topical ophthalmic use. Each mL contains 0.54 mg levocabastine hydrochloride equivalent to 0.5 mg levocabastine; 0.15 mg benzalkonium chloride; propylene glycol; polysorbate 80; dibasic sodium phosphate, anhydrous; monobasic sodium phosphate, monohydrate; disodium edetate; hydroxypropyl methylcellulose; and purified water. It has a pH of 6.0 to 8.0.
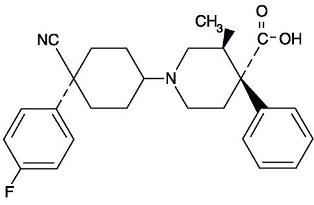
The chemical name for levocabastine hydrochloride is (–)-trans-1-[cis-4-Cyano-4- (p-fluorophenyl)cyclohexyl]-3-methyl-4-phenylisonipecotic acid monohydrochloride, and is represented by the following chemical structure:
 | ● HCl |
Livostin - Clinical Pharmacology
Levocabastine is a potent, selective histamine H1-antagonist.
Antigen challenge studies performed two and four hours after initial drug instillation indicated activity was maintained for at least two hours.
In an environmental study, Livostin™ 0.05% (levocabastine hydrochloride ophthalmic suspension) instilled four times daily was shown to be significantly more effective than its vehicle in reducing ocular itching associated with seasonal allergic conjunctivitis.
After instillation in the eye, levocabastine is systemically absorbed. However, the amount of systemically absorbed levocabastine after therapeutic ocular doses is low (mean plasma concentrations in the range of 1-2 ng/mL).
Indications and Usage for Livostin
Livostin™ 0.05% (levocabastine hydrochloride ophthalmic suspension) is indicated for the temporary relief of the signs and symptoms of seasonal allergic conjunctivitis.



