Locoid Cream
Generic name:hydrocortisone butyrate
Dosage form: cream
Drug class:Topical steroids
Medically reviewed by Drugs.com. Last updated on Sep 21, 2021.
On This Page
FOR TOPICAL USE ONLY
NOT FOR OPHTHALMIC USE
Locoid Cream Description
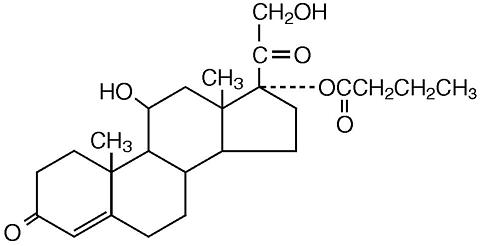
LOCOID® (hydrocortisone butyrate) cream, 0.1% contains the topical corticosteroid, hydrocortisone butyrate, a non-fluorinated hydrocortisone ester. It has the chemical name: 11ß,17,21-Trihydroxypregn-4-ene-3,20-dione 17-butyrate; the molecular formula: C25H36O6; the molecular weight: 432.54; and the CAS registry number: 13609-67-1.
Its structural formula is:
Each gram of Locoid Cream contains 1 mg of hydrocortisone butyrate in a hydrophilic base consisting of anhydrous citric acid, ceteth-20, cetostearyl alcohol, light mineral oil, propylparaben and butylparaben (preservatives), purified water, sodium citrate, and white petrolatum.
Locoid Cream - Clinical Pharmacology
Topical corticosteroids share anti-inflammatory, anti-pruritic, and vasoconstrictive actions. The mechanism of anti-inflammatory activity of topical corticosteroids is unclear. Various laboratory methods, including vasoconstrictor assays, are used to compare and predict potencies and/or clinical efficacies of topical corticosteroids. There is some evidence to suggest that a recognizable correlation exists between vasoconstrictor potency and therapeutic efficacy in man.
Pharmacokinetics
The extent of percutaneous absorption of topical corticosteroids is determined by many factors including the vehicle, the integrity of the epidermal barrier, and the use of occlusive dressings.
Topical corticosteroids can be absorbed from normal intact skin. Inflammation and/or other disease processes in the skin increase percutaneous absorption. Occlusive dressings substantially increase the percutaneous absorption of topical corticosteroids.
Once absorbed through the skin, topical corticosteroids are handled through pharmacokinetic pathways similar to systemically administered co...