Lustra Cream
Generic name:hydroquinone
Dosage form: cream
Drug class:Topical depigmenting agents
Medically reviewed by Drugs.com. Last updated on Jan 24, 2022.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
On This Page
LUSTRA®
(HYDROQUINONE CREAM USP, 4%)
The Complete Treatment for Dyschromia
LUSTRA-AF®
(HYDROQUINONE CREAM USP, 4%)
The Complete Treatment for Dyschromia
Rx Only
FOR EXTERNAL USE ONLY
The Lustra brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
I. DESCRIPTION
Hydroquinone is 1,4-benzenediol. Hydroquinone is structurally related to monobenzone. Hydroquinone occurs as fine, white needles. The drug is freely soluble in water and in alcohol and has a pKa of 9.96. Chemically, hydroquinone is designated as p-dihydroxybenzene; the empirical formula is C6H6O2; molecular weight 110.1. The structural formula is:
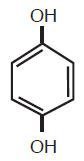
C6H6O2
CONTENTS
ACTIVE INGREDIENTHydroquinone USP 4%.
OTHER INGREDIENTS (LUSTRA®)Alcohol, benzyl alcohol, butylated hydroxytoluene, carbomer 940, cetearyl alcohol (and) ceteareth-20, cetyl alcohol, cyclopentasiloxane (and) polysilicone-11, dimethiconol, disodium EDTA, fragrance, glycerin, glyceryl stearate (and) PEG-100 stearate, glycolic acid, hydrogenated lecithin, linoleic acid, magnesium 1-ascorbyl phosphate, phenoxyethanol, phenyl trimethicone, polyacrylamide (and) C13-14 isoparaffin (and) laureth 7, purified water, ...



