Lyleq
Generic name:norethindrone
Dosage form: tablet
Drug classes:Contraceptives, Progestins
Medically reviewed by Drugs.com. Last updated on Jan 1, 2021.
On This Page
Patients should be counseled that oral contraceptives do not protect against transmission of HIV (AIDS) and other sexually transmitted diseases (STDs ) such as Chlamydia, genital herpes, genital warts, gonorrhea, hepatitis B, and syphilis.
Lyleq Description
Each light yellow to yellow LyleqTM tablet provides a continuous oral contraceptive regimen of 0.35 mg norethindrone USP daily, and the inactive ingredients include corn starch, D&C Yellow No. 10 aluminum lake, FD&C Yellow # 6 aluminum lake, lactose monohydrate, magnesium stearate, povidone and talc.
The chemical name for norethindrone is 17-Hydroxy-19-Nor-17α-pregn-4-en-20-yn-3-one. The structural formula follows: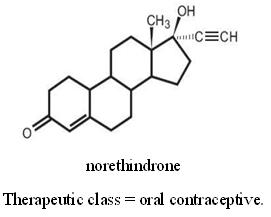
Lyleq - Clinical Pharmacology
1. Mode of Action
Lyleq progestin-only oral contraceptives prevent conception by suppressing ovulation in approximately half of users, thickening the cervical mucus to inhibit sperm penetration, lowering the mid-cycle LH and FSH peaks, slowing the movement of the ovum through the fallopian tubes, and altering the endometrium.
2. Pharmacokinetics
Absorption
Norethindrone is rapidly absorbed with maximum plasma concentrations occurring within 1 to 2 hours after Lyleq administration (see Table 1). Norethindrone appears to be completely absorbed following oral administration; however, it is subject to first pass metabolism resulting in an absolute bioavailability of approximately 65%.
Figure 1: Mean ± SD Norethindrone Plasma Concentrations Following Lyleq Administration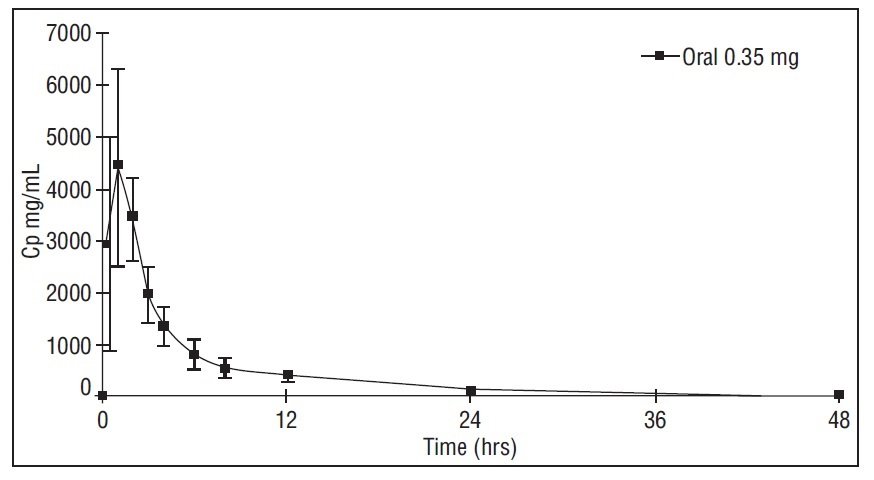
Peak plasma concentrations occur approximately 1 hour after administration (mean Tmax 1.2 hours). The mean (SD) Cmax was 4816.8 (1532.6) pg/mL a



