Medicated Buccal DNA Collection Kit
Generic name: lidocaine hydrochloride
Dosage form: kit
On This Page
A Topical Anesthetic for the Mucous Membranes of the Mouth and Pharynx.
Rx Only
For Oral Use Only
Postmarketing cases of seizures, cardiopulmonary arrest, and death in patients under the age of 3 years have been reported with use of Lidocaine Hydrochloride Oral Topical Solution, USP (Viscous) 2% when it was not administered in strict adherence to the dosing and administration recommendations. In the setting of teething pain, Lidocaine Hydrochloride Oral Topical Solution, USP (Viscous) 2% should generally not be used. For other conditions, the use of the product in patients less than 3 years of age should be limited to those situations where safer alternatives are not available or have been tried but failed.
To decrease the risk of serious adverse events with use of Lidocaine Hydrochloride Oral Topical Solution, USP (Viscous) 2%, instruct caregivers to strictly adhere to the prescribed dose and frequency of administration and store the prescription bottle safely out of reach of children.
DESCRIPTION
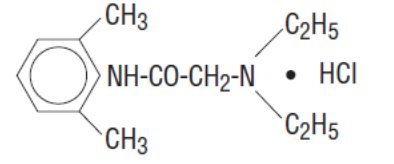
Lidocaine Hydrochloride Oral Topical Solution, USP (Viscous) 2% contains a local anesthetic agent and is administered topically. Lidocaine Hydrochloride Oral Topical Solution, USP (Viscous) 2% contains lidocaine hydrochloride, which is chemically designated as acetamide, 2-(diethylamino)-N- (2,6 dimethylphenyl)-, monohydrochloride and has the following structural formula:
The molecular formula of lidocaine is C 14H 22N 2O. The molecular weight is 234.34.
COMPOSITION OF SOLUTION
Each mL contains 20 mg of lidocaine HCl. In addition each mL contains the following inactive ingredients: Carboxymethylcellulose sodium, methylparaben, natural orange flavor, propylparaben, purified water, and saccharin sodium. The pH is adjusted to 5.0 to 7.0 by means of hydrochloric acid and/or sodium hydroxide.