Medolor Pak
Generic name:lidocaine and prilocaine
Dosage form: kit
Drug class:Topical anesthetics
Medically reviewed by Drugs.com. Last updated on Nov 22, 2021.
On This Page
The Medolor Pak brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
Medolor Pak (Lidocaine 2.5% and Prilocaine 2.5%) Cream
For Topical Use Only. Not for Ophthalmic Use.
Medolor Pak Description
Lidocaine 2.5% and Prilocaine 2.5%, a topical anesthetic agent, is an emulsion in which the oil phase is a eutectic mixture of lidocaine and prilocaine in a ratio of 1:1 by weight. This eutectic mixture has a melting point below room temperature and therefore both local anesthetics exist as a liquid oil rather than as crystals. It is packaged in 15 gram and 30 gram tubes.
Lidocaine is chemically designated as acetamide, 2-(diethylamino)-N-(2, 6-dimethylphenyl), has an octanol:water partition ratio of 43 at pH 7.4, and has the following structure:
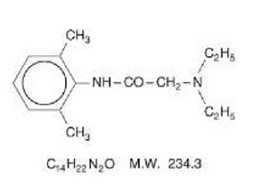
Prilocaine is chemically designated as propanamide, N-(2-methylphenyl)-2-(propylamino), has an octanol:water partition ratio of 25 at pH 7.4, and has the following structure:
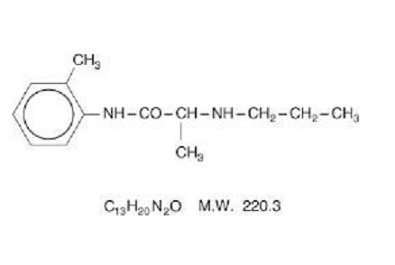
Each gram of lidocaine 2.5% and prilocaine 2.5% cream contains lidocaine 25 mg, prilocaine 25 mg, carboxypolymethylene (as a thickening agent), polyoxyethylene fatty acid esters (as emulsifiers), purified water to 1 gram, and sodium hydroxide to adjust pH (pH range 9.0-9.4). Lidocaine 2.5% and prilocaine 2.5% cream contains no preservative, however it passes the USP antimicrobial effectiveness test due to the pH. The specific gravity of lidocaine 2.5% and prilocaine 2.5% cream is 1.00.
Medolor Pak - Clinical Pharmacology
Mechanism of Action: Lidocaine 2.5% and prilocaine 2.5% cream, applied to intact skin under occlusive dressing, provides dermal analgesia by the release of lidocaine and prilocaine from the cream into the epidermal and dermal layers of the skin and by the accumulation of .



