Metaproterenol Syrup
Generic name: metaproterenol sulfate
Dosage form: oral syrup
Drug class:Adrenergic bronchodilators
Medically reviewed by Drugs.com. Last updated on May 23, 2022.
On This Page
Metaproterenol Syrup Description
Metaproterenol sulfate syrup is an oral bronchodilator.
Each teaspoonful (5 mL), for oral administration, contains metaproterenol sulfate 10 mg. In addition, each teaspoonful (5 mL) contains the following inactive ingredients:
citric acid, edetate disodium, FD&C Red No. 40, glycerin, hydroxyethyl cellulose, black cherry flavor, propylene glycol, saccharin sodium, sodium benzoate, sorbitol solution, sodium citrate and purified water.
Metaproterenol sulfate, 1- (3, 5 dihydroxyphenyl) -2-isopropyl - aminoethanol sulfate, is a white, crystalline, racemic mixture of two optically active isomers. It has the following structural formula:
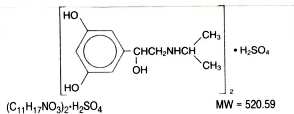
Metaproterenol Syrup - Clinical Pharmacology
In vitro studies and in vivo pharmacologic studies have demonstrated that metaproterenol sulfate has a preferential effect on beta2 adrenergic receptors compared with isoproterenol. While it is recognized that beta2 adrenergic receptors are the predominant receptors in bronchial smooth muscle, recent data indicate that there is a population of beta2 receptors in the human heart existing in a concentration between 10 to 50%. The precise function of these, however, is not yet established (see WARNINGS section).
The pharmacologic effects of beta adrenergic agonist drugs, including metaproterenol, are at least in part attributable to stimulation through beta adrenergic receptors of intracellular adenyl cyclase, the enzyme which catalyzes the conversion of adenosine triphosphate (ATP) to cyclic-3’, 5’ -adenosine monophosphate (c-AMP). Increased c-AMP levels are associated with relaxation of bronchial smooth muscle and inhibition of release of mediators of immediate hypersensitivity from cells, especially from mast cells.
PHARMACOKINETICS: Absorption, biotransformation and excretion studies in humans following oral administration h...



