Dosage form: injection, powder, lyophilized, for solution
Drug class:Glucocorticoids
Medically reviewed by Drugs.com. Last updated on Jan 1, 2022.
On This Page
After mixing as directed, contains benzyl alcohol. Not for use in neonates.
For Intravenous or Intramuscular Administration
Methylprednisolone Sodium Succinate Injection Description
Methylprednisolone sodium succinate for injection, USP is an anti-inflammatory glucocorticoid, which contains methylprednisolone sodium succinate as the active ingredient. Methylprednisolone sodium succinate, USP, is the sodium succinate ester of methylprednisolone, and it occurs as a white, or nearly white, odorless hygroscopic, amorphous solid. It is very soluble in water and in alcohol; it is insoluble in chloroform and is very slightly soluble in acetone.
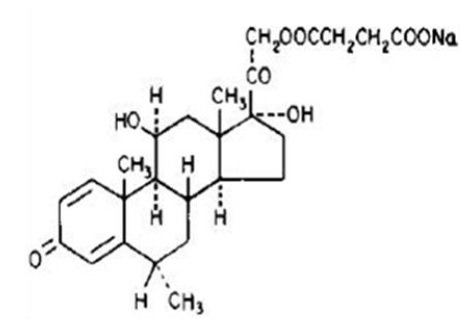
The chemical name for methylprednisolone sodium succinate is pregna-1,4-diene-3,20-dione, 21-(3-carboxy-1-oxopropoxy)-11, 17-dihydroxy-6-methyl-monosodium salt, (6α, 11β), and the molecular weight is 496.53. The structural formula is represented below:
Methylprednisolone sodium succinate is soluble in water; it may be administered in a small volume of d..



