Proctocort
Generic name:hydrocortisone acetate
Dosage form: suppository
Drug class:Topical steroids
Medically reviewed by Drugs.com. Last updated on Mar 22, 2022.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. Read further information about unapproved drugs.
On This Page
Proctocort Description
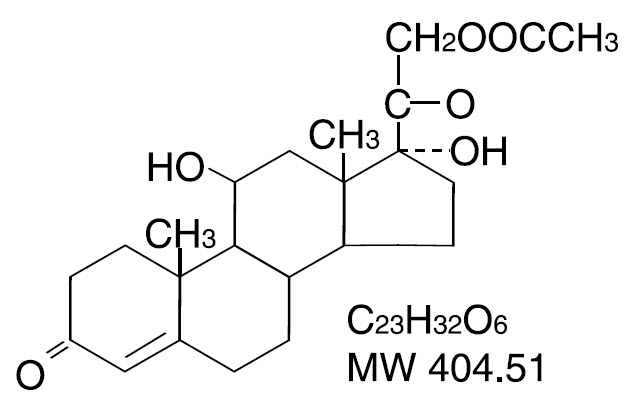
Hydrocortisone Acetate is a corticosteroid designated chemically as pregn-4-ene-3, 20-dione, 21-(acetyloxy)-11, 17-dihydroxy-(11β) with the following structural formula:
Each rectal suppository contains hydrocortisone acetate, USP 30 mg in a specially blended hydrogenated vegetable oil base.
Proctocort - Clinical Pharmacology
In normal subjects, about 26% of hydrocortisone acetate is absorbed when the suppository is applied to the rectum. Absorption of hydrocortisone acetate may vary across abraded or inflamed surfaces. Topical steroids are primarily effective because of their anti-inflammatory, anti-pruritic and vasoconstrictive action.
Indications and Usage for Proctocort
Proctocort® Suppositories are indicated for use in inflamed hemorrhoids, postirradiation (factitial) proctitis; as an adjunct in the treatment of chronic ulcerative colitis; cryptitis; and other inflammatory conditions of anorectum and pruritus ani.
Contraindications
Proctocort® Suppositories are contraindicated in those patients having a history of hypersensitivity to hydrocortisone acetate or any of the components.
Precautions
Do not use Proctocort® Suppositories unless adequate proctologic examination is made.
If irritation develops, the product should be discontinued and appropriate therapy instituted.
In the presence of an infection, the use of an appropriate antifungal or anti-bacterial agent should be instituted. If ...